I
iTeachChem
A Study Community for Everyone and Everything.We have weekly sessions on Spirituality, Math and Science! JEE, NEET prep? All doubts are crowdsourced. Hare Krishna!
JoinI
iTeachChem
A Study Community for Everyone and Everything.We have weekly sessions on Spirituality, Math and Science! JEE, NEET prep? All doubts are crowdsourced. Hare Krishna!
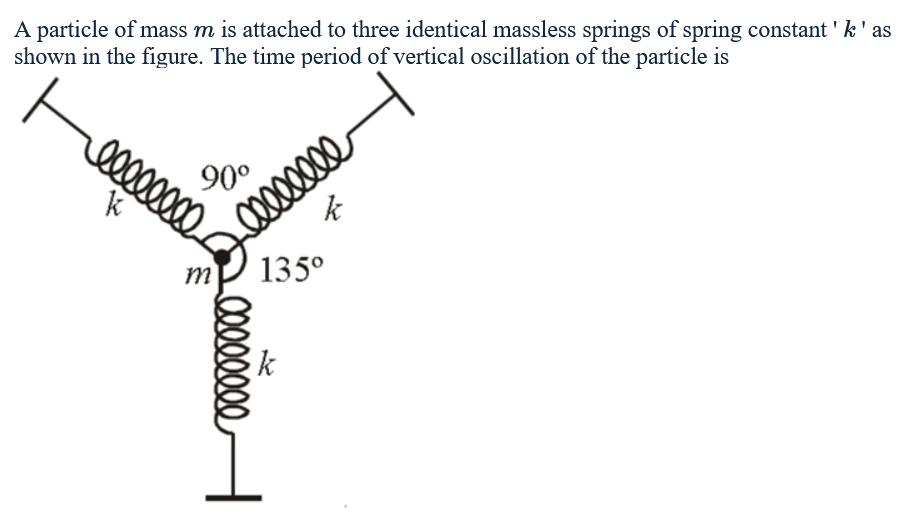
JoinSHM, time period of spring system
I guess I'm having trouble in the geometry? Not sure. How do we find K_equivalent?
Ans is 2pi * sqrt(m/2K)...
Respiration
It says 'of isocitrate', so why do they consider the decarboxylation of alpha-ketoglutaric acid, just because it comes after isocitrate? Most the coaching answer keys say it's option B, but I checked the official key and it's option C. Sometimes I have trouble understanding NTA's brand of logic. Should we apply this understanding of the term to future questions?

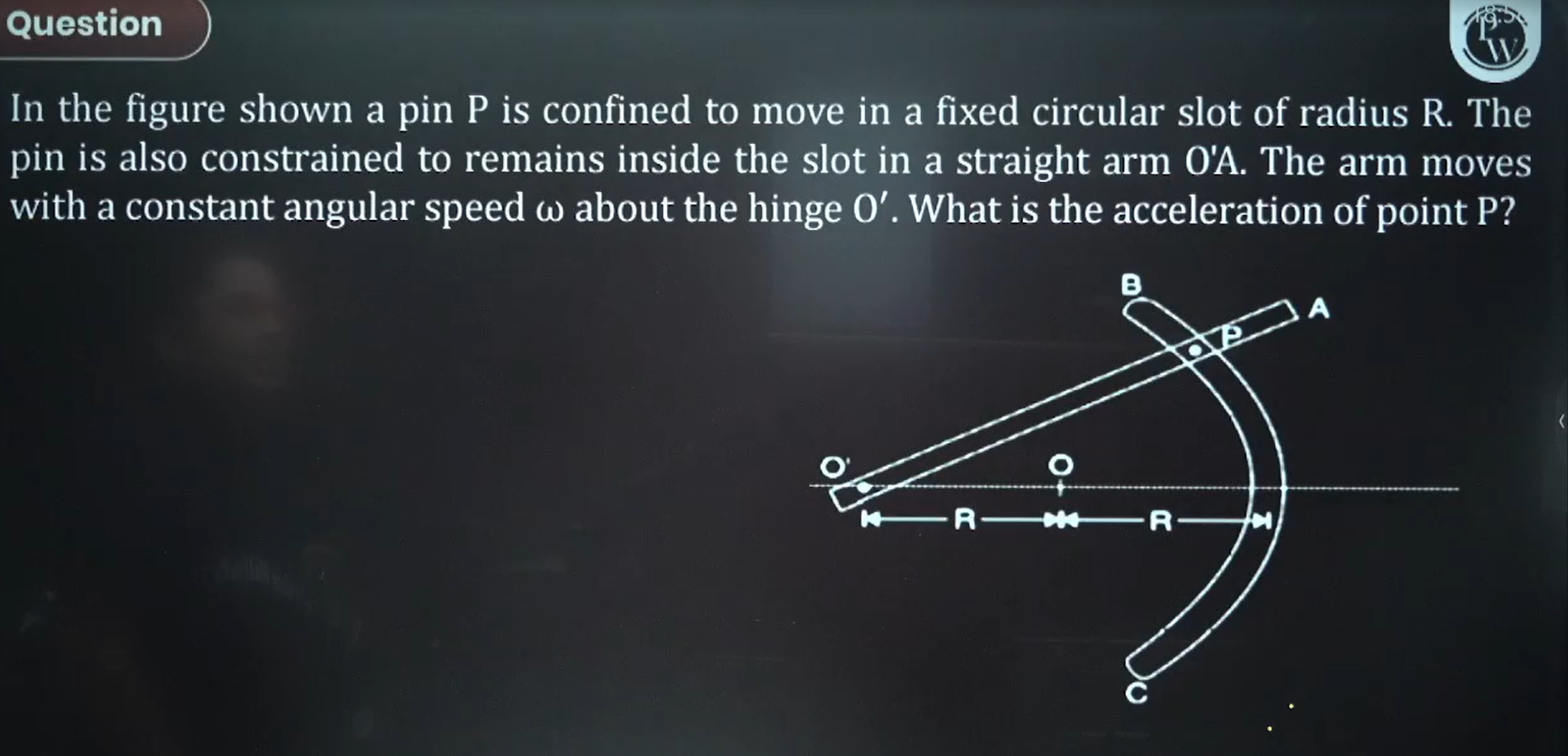
Circular Motion
Since w is constant, alpha = 0...so there is no tangential acceleration, only centripetal acceleration. Should we consider rotational motion about O or about O'?
Jee Adv Answering
i got the ans for this quest , Q1 = 4/3 , Q2 = 2/3
but it is numerical how will we even answer?
like we just write 1.3 for Q1 and 0.6 for Q2?
what if we write 0.67 for Q2 how does adv mark these questions...

dipole
yeah im still not able to solve que like this
three point charges +q,-2q and +q are placed at points (x=0,y=a,z=0), (x=0,y=0,z=0) and (x=a,y=0,z=0 respectively. the magnitude and direction of e;ectric dipole moment vector of this charge assembly are...
silly sn1,2 doubt
the teacher said we would get sn1 and sn2 at same position just sn1 product wont show optical activity and sn2 one will...but in sn1 carbocation should rearrange to allylic position right?

Limits LHL and RHL Related doubt
So My Sir directly found the LHL and RHL of the limit while i used h tends to 0 method. Is there any method/trick to directly find LHL and RHl ?

Electric dipole
how do we solve the ques which have the dipole distributed in an eqilateral triangle like the ques that go electric charge q,q 2q are placed on vertices of equilateral triangle calculate effective dipole moment
capacitance
so in the 3rd diagram our teacher said we can ignore the c5 when c1/c3=c2/c4
my question is how ? like i didnt understood how we can and if in more such future circuits how will i know tht i have to skip tht capacitor...

Zumdahl kineticsmarathon
Idk ismein marathon wale question mein first table or second table dono hin bilkul alag se lag rhen h...dono ka r.o.r. hin alag h...so idk how to even get at the first question done...sorry feeling dumb 😕

Capacitor
it says battery disconnected then shouldnt voltage remain const , charge will be doubled and work will be approx 4.4 x 10^-6?









