I
iTeachChem
A Study Community for Everyone and Everything.We have weekly sessions on Spirituality, Math and Science! JEE, NEET prep? All doubts are crowdsourced. Hare Krishna!
JoinI
iTeachChem
A Study Community for Everyone and Everything.We have weekly sessions on Spirituality, Math and Science! JEE, NEET prep? All doubts are crowdsourced. Hare Krishna!
JoinPolynomial
I'm using Descartes rule of signs, and getting 1 sign change in f(x) and 4 sign changes in f(-x) meaning 5 real roots so acc to me then sum of non real roots should be 0, but that's not correct.
So what's the mistake?...

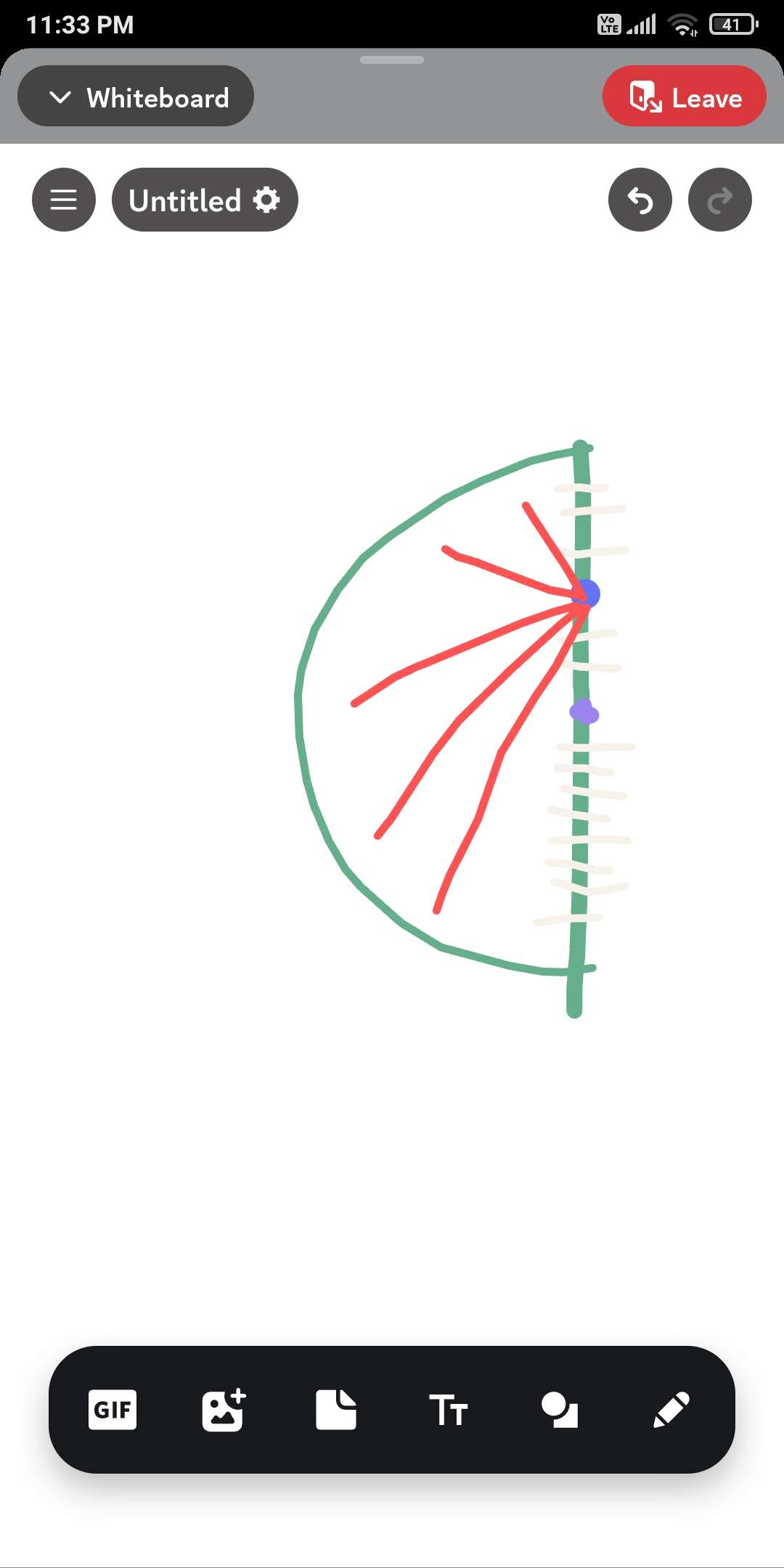
Electrostat
To ye semicircular ring h uniform charge density ki and mujhe jo blue pint h uske about nikalni h field. Lets say ki blue point ka altitude given
To fir iske upar field kese nikalenge. I thought of integrating the e vectors depicted in red but distance is also changing .
Could also look at it like a bended rod...
Solids/waves, frequency of a bar
A uniform metal rod of length 1m is clamped at two points 5 cm from one end and 15 cm from the other end. The possible frequencies of the natural oscillations (longitudinal) of the rod can be (Y=1.6×1011 N/m2, ρ=2500 kg/m3)
A uniform metal rod of length 1m is clamped at two points 5 cm from one end and 15 cm from the other end. The possible frequencies of the natural oscillations (longitudinal) of the rod can be (Y=1.6×1011 N/m2, ρ=2500 kg/m3)

Rotation, polygon rolling
Consider regular polygons with number of sides n=3,4,5… as shown in the figure. The center of mass of all the polygons is at height h from the ground. They roll on a horizontal surface about the leading vertex without slipping and sliding as depicted. The maximum increase in height of the locus of the center of mass for each polygon is Δ . Then, Δ depends on n and h as
Consider regular polygons with number of sides n=3,4,5… as shown in the figure. The center of mass of all the polygons is at height h from the ground. They roll on a horizontal surface about the leading vertex without slipping and sliding as depicted. The maximum increase in height of the locus of the center of mass for each polygon is Δ . Then, Δ depends on n and h as

gauss law
a hollow insulating sphere contains a point charge +q are its centre, find force between them
Derivation doubt
I think the result given in the book (cengage) is incorrect. I've attached my own derivation below

Electric potential doubt
Check the image attached. For electric potential when r< a, we are considering potential due to 1) charge at centre 2) induced charges
For induced charges, why have we taken the distance from the centre of sphere (a,b) and why not (a-r,b-r)...

Spectral Lines
The answer for this question is given as 4
But I thought that for these type of questions we should use the formula: Max spectral lines = (n2-n1)(n2-n1+1)/2...

Mole concept - PLS help
Q- (100gm of CuSO4 . 5 H2O is dissolved in 500 g of water to form aq solution. find molarity & molality of Cu2+ if density of solution is 1.2 g/ml [Cu=64] [S=96])
isme sir ne bola water of crystallisation ka bhi mass lena hoga molality find krne ke liye or usme unhone water of crystallisation ke 2.5 mole liye , ye smajh nhi aaya...









