8 Replies
@Dexter
Note for OP
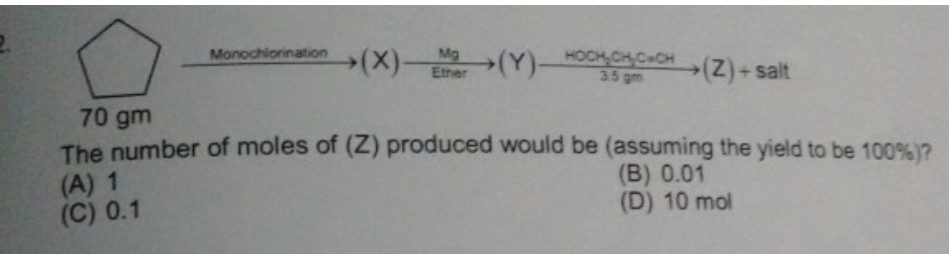
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.reagent is 3.5gm ohch2ch2cch if not clear
It has an alcohol group so just a proton donor
So you get the original compound again
then 0.05 shouldve been the answer??
Mb, terminal alkyne, so two moles of H(+) per mole reagent
Both the OH and the ≡CH donate a H(+)
why
what even is that rxn btw
Grignard reagent with a proton donating species gives you the hydrocarbon corresponding to Grignard reagent
RMgX → R(-)
R(-) + H(+) → RH
Triple bonded carbon is very electronegative. The hydrogen attached to it barely shares any of the electron density, and thus, can be donated as a proton