15 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.
how does one infer the second thing here going backwards
following it backwards, i thought it was -OH on top of the 5-ring with a still intact 5-ring
ill draw it hang on
my inference of compound 2
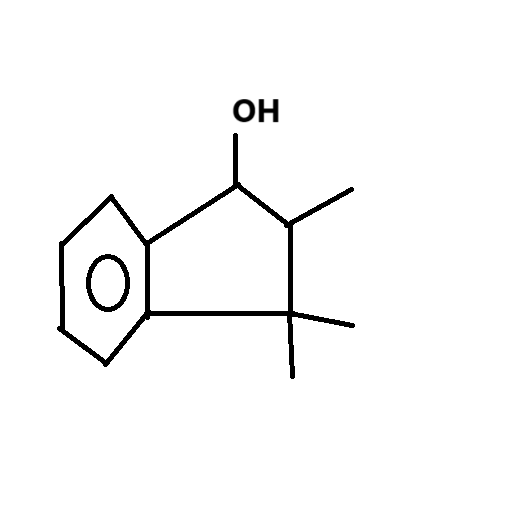
heating -OH makes the double bond go onto the same position as compound number (3) here, goes it not?
It does, yeah, but see that there is no methyl group connected to the C-OH, which would be a necessity assuming you prepared the alcohol with an aldehyde and methyl magnesium bromide
Which means that you initially had a tertiary alcohol
Meaning your original compound was a ketone
ah shiyet
that's true
also, why are acidic hydrolysis and conc,H2SO4/heat listed as separate steps?
even in the solution they only consider H+ and heat
The hydrolysis is for the magnesium complex to be split into an organic compound and a magnesium salt.
Oh I took that for granted
No, it isn't.
?
It sounds correct once you said it
Then the second conc.H2SO4 protonates OH and abstracts H2O, generating electrophile (carbocation). No rearrangement since it's already tertiary, so EAS happens
Ortho instead of meta because of bond length constraints.
Yeah got that part
Thanks for solving a lot of my doubts by the way
+solved opt314
Post locked and archived successfully!
Archived by
<@717724055217635398> (717724055217635398)
Time
<t:1733578657:R>
Solved by
<@763645886500175892> (763645886500175892)
