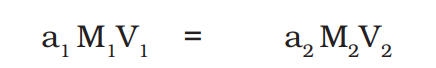titration
so yeah, I did titration with KMnO4 and mohr's salt solution today in which I need to find the molarity and percentage purity of the KMnO4 solution when the molarity of mohr's salt solution is M/40 and here is the observation and calculation I did (attached). I got percentage purity about 105% I mean what!? tell me if it's true or where I fucked up.