Screening Effect
I am a little confused between these terms>
Shielding effect,screening effect,sheilding power,screening power
Are all these terms same?
Do the inner electrons absorb or suck up the nuclear force of attraction by some extent or they just repel the test electron or they do both?
does shielding power mean the the strength or capacity of inner electrons to suck up or decrease effective nuclear charge?
9 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.read whole message please><><><
Shielding Effect Screening Effect same thing and last two are same thing
more than electrons it has to do with orbitals more specifically shape of orbitals
u see these orbitals shape the S orbital electrons are everywhere so they "Screen" the force the best
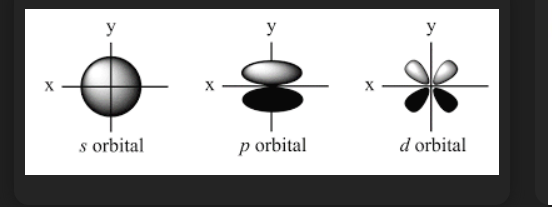
so how do they screen like they dont let the full force get out of it (say s orbital)?
whats the diff between shielding and screening effect/power?
its kind of complicated since classical mechanics does not apply
The Phenomena by which the force on last shell e is reduced is called screening effect
And how much the force reduces is decided by power so S has highest Screening Power
+solved @Gamertug thanks a lot mate
Post locked and archived successfully!
Archived by
<@1179817028106858538> (1179817028106858538)
Time
<t:1723571222:R>
Solved by
<@700658749416669194> (700658749416669194)