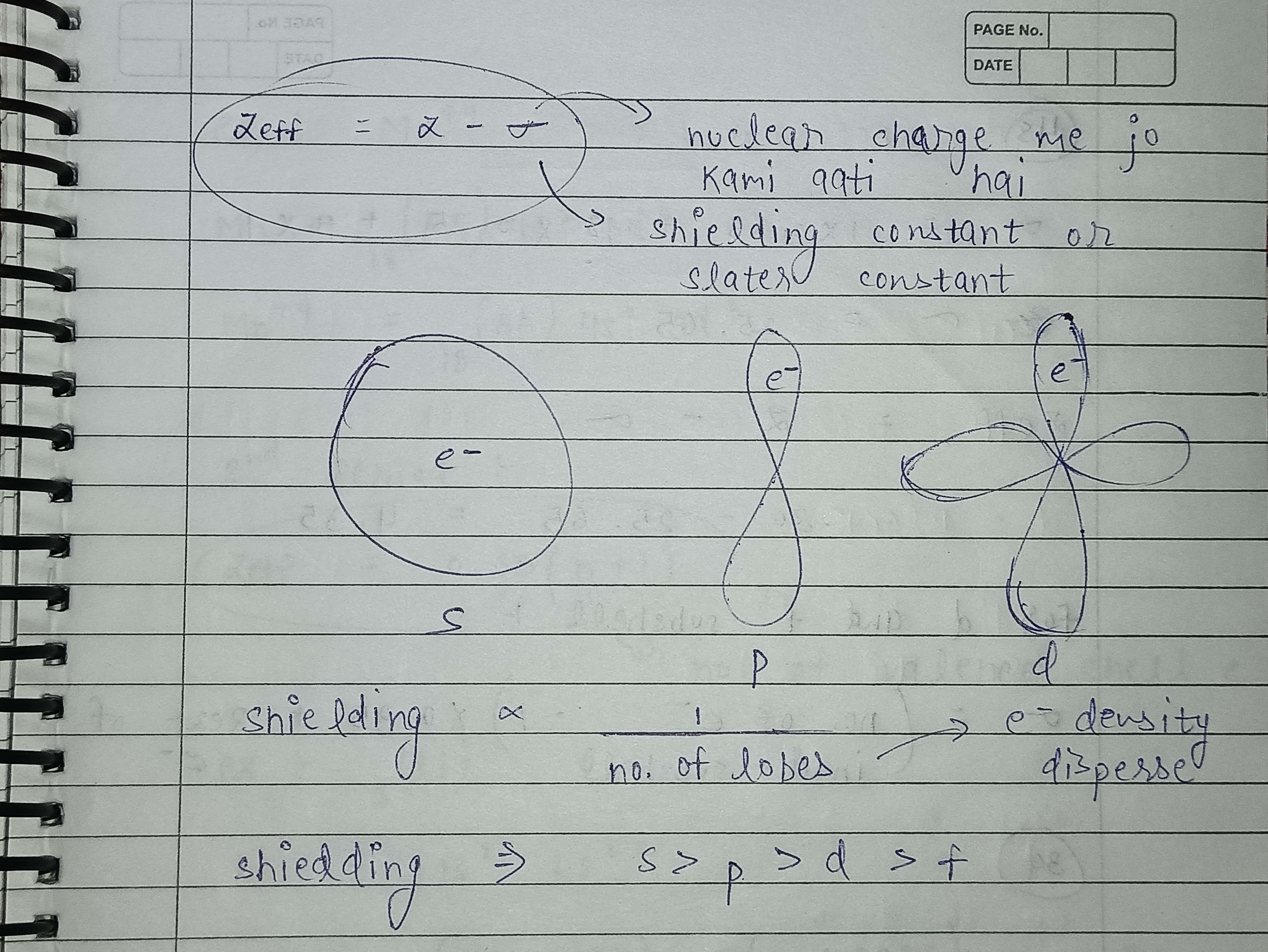
Shielding Constant
I don't understand how the shape of an orbital can affect the shielding constant
31 Replies
@Dexter
Note for OP
+solved @user to close the thread when your doubt is solved. Mention the user who helped you solve the doubt. This will be added to their stats.Can you reference this from the book? where it says shielding is indirectly proportional to number of lobes.
I tried finding, was not able to
Got no book. These are the notes from my teacher's class.
Kuch bhi likhwa dete hai sir 😭
It is not relent also, it is just mere coincidence that more lobes = less shielding, the main factor is electron penetration in orbital. as per my knowledge
with the same criteria, d(z^2) orbital will be as powerfull as p shell cause 2 lobes hi hote hai usme. doesnt make sense
Forget lobes. Just give me a simple explanation on why electrons in s - orbitals are better at shielding than f - orbital electrons.
Less dispersed?
i'm a depressed.......CUBIC
🤔
This is right too
electron penetration
So the basic funda is that the core electrons are better at shielding because they are closer to the nucleus.
See the thing is, An electron in S shell can get close to nucleus easily, so there is more probability of the electron that is may be present near nucleus in the electron cloud, hence it will shield more effectively,
P have less penetration power, the electron struggles to go near nucleus
Same for D and F but more
I see
An 2S electron can be found in 1S cloud too, but finding a 2P electron near 1s shell is rare
Because it's a different shell all together?
no, it's because shape of P orbital, there is some probability that 2P electron may be found in 1s cloud too, but its very rare
Acha

visualize with this diagram
Chemistry LibreTexts
7.2: Shielding and Effective Nuclear Charge
The calculation of orbital energies in atoms or ions with more than one electron (multielectron atoms or ions) is complicated by repulsive interactions between the electrons. The concept of electron …
Understood

See the diff bw Ne and Na
I think the nodes create a problem, which is why it is rare to find an electron in the intersection of both regions
this should help
node just means no chance to find electron
they have nodal planes btw (p block)
p orbitals*
Yeah

The outermost electron is further away from the nucleus due to the introduction of a new shell. Which is why sodium has less effective nuclear charge in comparison to neon.
I think.
Looks fun but I'm not able to understand the figures beyond n = 3 😂
Got the crux of the idea after reading this. Thanks sir!
+solved @atamakahere
Post locked and archived successfully!
Archived by
<@624803801173327923> (624803801173327923)
Time
<t:1717420863:R>
Solved by
<@270847109521997824> (270847109521997824)