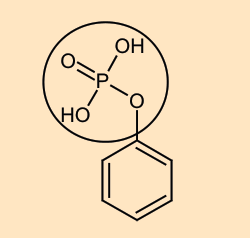
GOC
How is the circled group showing -I effect?
I know O is quite EN .
But O would already get a negative induced charge due to polarisatiom between P and O. So, won't its electron withdrawing tendency decrease?
6 Replies
@Dexter
Note for OP
+solved @user to close the thread when your doubt is solved. Mention the user who helped you solve the doubt. This will be added to their stats.I believe it's a weak -I effect
O is attached to phosphorus and sp2 hybridised carbon which are both less electronegative than it
Hence most of the negative charge is pulled closer to oxygen
its a weak acid. hence it cant show -R / -M effect. but quite possible it may show a weak -I effect because instead of 3 H there are only 2H attached to O , so its basicity might have decreased and acidity might have increased slightly
+solved @Comrade Rock Astley @seksosterone
Post locked and archived successfully!
Archived by
<@1082354613749035099> (1082354613749035099)
Time
<t:1715336302:R>
Solved by
<@769492044837552139> (769492044837552139)